Abstract
Background: In many solid cancers, increased numbers of intra-tumoral neutrophils are associated with poor prognosis. Interestingly, and in contrast to peripheral tissues, the bone marrow (BM) is unique in its continuous occupation by high numbers of neutrophils, making these cells likely candidates to influence the tumor microenvironment in BM malignancies such as multiple myeloma (MM). Accordingly, a high neutrophil/T-cell ratio is associated with inferior progression-free survival in MM. Recently, we identified inflammatory mesenchymal stromal cells (iMSC) in the MM BM that transcribe myeloid modulation factors, including chemokines that bind the neutrophil-expressed receptors CXCR1 and 2. This led us to hypothesize that neutrophil-stromal cell crosstalk in MM might lead to neutrophil alterations and by virtue of their abundance, the creation of a pro-tumor MM environment.
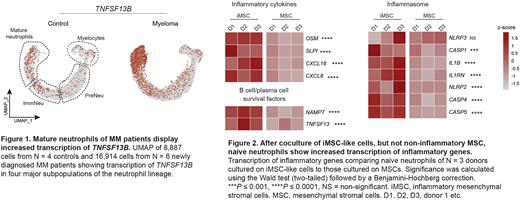
Results: To determine whether BM neutrophils of MM patients differed from those of controls, we first assessed phenotypic alterations by flow cytometry. Compared to non-cancer controls, mature neutrophils from the MM BM had an activated phenotype, evidenced by increased numbers of CD62L negative cells (37% vs. 5%, p = 0.03, N = 5 newly diagnosed MM, 3 controls), switching of CD11b to its activated isoform (74% vs. 17%, p = 0.03), and upregulation of CXCR1 (mean fluorescent intensity [MFI] 7574 vs. 1831, p = 0.03) and CXCR2 (MFI 2284 vs. 813, p = 0.03). To assess the extent of activation, we performed single cell RNA sequencing of the entire BM granulocytic lineage (N = 6 MM, 4 controls). This confirmed the activation status of mature neutrophils in MM, and suggested an association with inflammation, as mature neutrophils of patients with MM were characterized by increased transcription of inflammatory mediators such as CXCL8, OSM and SLPI, inflammasome components IL1B, NLRP3 and CASP1, and various toll-like receptors (TLRs). One of the most prominent genes differentially expressed in mature neutrophils from MM patients was TNFSF13B, which encodes the MM cell survival factor BAFF (Figure 1). A transcriptomic comparison of TNFSF13B levels across hematopoietic and non-hematopoietic cells from the BM revealed that transcription of this gene was highest in mature neutrophils found in the MM BM. Combined with the fact that mature neutrophils are the most abundant hematological cells in the MM BM (37.7 ± 9% of all nucleated cells, N = 3), this finding suggests that neutrophils are a major source of BAFF in MM.
As iMSC transcribe many factors with the potential to modulate neutrophils, we hypothesized that neutrophilic activation was mediated by iMSC. Coculture with IL1β-activated iMSC-like cells, but not non-inflammatory MSCs, led to activation of naïve neutrophils, as evidenced by the acquisition of a surface protein expression profile almost identical to that of BM neutrophils from MM patients. Moreover, RNA-sequencing of neutrophils cocultured with iMSC revealed increased transcription of inflammatory genes as observed in MM BM neutrophils (Figure 2). Importantly, interactions between naïve neutrophils and iMSC resulted in release of BAFF protein by neutrophils (29pg/mL vs. 15pg/mL on MSC, p = 0.008), suggesting that stromal cell-neutrophil interactions can drive a pro-MM environment by activation of local neutrophils.
Finally, we assessed the durability of neutrophilic activation in patients undergoing first-line treatment. Single cell RNA sequencing of BM neutrophils from patients after ASCT and consolidation treatment revealed persistent neutrophil activation including high transcription of TNFSF13B. This was supported by increased levels of BAFF protein in BM plasma of MM patients post-consolidation compared to controls (3541 pg/mL vs. 411 pg/mL, p <0.0001, N = 52 MM and 18 controls). To assess if neutrophil activation and increased BAFF levels persist long-term, we determined levels of BAFF in BM supernatants of patients 1 year after consolidation therapy that did (N = 33) or did not (N = 19) receive daratumumab maintenance. Intriguingly, even after 1 year of maintenance therapy, and even in patients that were still MRD negative, protein levels of BAFF remained elevated (1096 pg/mL, p <0.0001).
Conclusion: The continued presence of activated and BAFF-producing BM neutrophils during treatment creates a MM-supportive environment with the potential to impact malignant plasma cell survival and disease recurrence.
Disclosures
Langerak:Genentech: Research Funding; Gilead: Speakers Bureau; Janssen: Speakers Bureau; Roche: Research Funding. Broijl:Janssen: Other: Advisory boards/honoraria; Sanofi: Other: Advisory boards/honoraria; Amgen: Other: Advisory boards/honoraria; Bristol Myers Squibb: Other: Advisory boards/honoraria. Sonneveld:Karyopharm: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal